What if I Stop Taking Metronidazole Gel Then Started Again
 | |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 80% (by mouth), threescore–80% (rectal), 20–25% (vaginal)[ane] [ii] [3] |
| Protein binding | 20%[1] [2] |
| Metabolism | Liver[ane] [2] |
| Metabolites | Hydroxymetronidazole |
| Emptying one-half-life | viii hours[i] [ii] |
| Excretion | Urine (77%), faeces (14%)[1] [2] |
| Identifiers | |
| IUPAC name
| |
| CAS Number |
|
| PubChem CID |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| NIAID ChemDB |
|
| PDB ligand |
|
| CompTox Dashboard (EPA) |
|
| ECHA InfoCard | 100.006.489 |
| Chemical and physical data | |
| Formula | C 6 H 9 N three O 3 |
| Molar mass | 171.156 m·mol−i |
| 3D model (JSmol) |
|
| Melting signal | 159 to 163 °C (318 to 325 °F) |
| SMILES
| |
| InChI
| |
| (verify) | |
Metronidazole, marketed under the brand proper noun Flagyl among others, is an antibiotic and antiprotozoal medication.[iv] It is used either lone or with other antibiotics to treat pelvic inflammatory disease, endocarditis, and bacterial vaginosis.[four] It is effective for dracunculiasis, giardiasis, trichomoniasis, and amebiasis.[four] Information technology is an pick for a offset episode of balmy-to-moderate Clostridium difficile colitis if vancomycin or fidaxomicin is unavailable.[4] [5] Metronidazole is available by mouth, as a cream, and past injection into a vein.[4]
Common side effects include nausea, a metallic taste, loss of appetite, and headaches.[4] Occasionally seizures or allergies to the medication may occur.[4] Some state that metronidazole should not be used in early pregnancy, while others state doses for trichomoniasis are safe.[vi] Metronidazole is generally considered uniform with breastfeeding.[half dozen] [seven]
Metronidazole began to be commercially used in 1960 in French republic.[viii] It is on the Earth Health Organization's List of Essential Medicines.[ix] Information technology is bachelor in nigh areas of the earth.[10] In 2019, it was the 138th well-nigh commonly prescribed medication in the Usa, with more than ivmillion prescriptions.[eleven] [12]
Medical uses [edit]

Metronidazole is primarily used to treat: bacterial vaginosis, pelvic inflammatory affliction (along with other antibacterials like ceftriaxone), pseudomembranous colitis, aspiration pneumonia, rosacea (topical), fungating wounds (topical), intra-abdominal infections, lung abscess, periodontitis, amoebiasis, oral infections, giardiasis, trichomoniasis, and infections caused by susceptible anaerobic organisms such as Bacteroides, Fusobacterium, Clostridium, Peptostreptococcus, and Prevotella species.[xiii] It is also often used to eradicate Helicobacter pylori along with other drugs and to prevent infection in people recovering from surgery.[xiii]
Metronidazole is biting and so the liquid suspension contains metronidazole benzoate. This may require hydrolysis in the gastrointestinal tract and some sources speculate that it may be unsuitable in people with diarrhea or feeding-tubes in the duodenum or jejunum.[14] [fifteen]
Bacterial vaginosis [edit]
Drugs of selection for the treatment of bacterial vaginosis include metronidazole and clindamycin. The treatment of choice for bacterial vaginosis in nonpregnant women include metronidazole oral twice daily for seven days, or metronidazole gel intravaginally once daily for five days, or clindamycin intravaginally at bedtime for vii days. For pregnant women, the handling of selection is metronidazole oral three times a mean solar day for seven days. Data does non report routine treatment of male sexual partners.[16]
Trichomoniasis [edit]
The 5-nitroimidazole drugs (metronidazole and tinidazole) are the mainstay of treatment for infection with Trichomonas vaginalis. Treatment for both the infected patient and the patient'south sexual partner is recommended, even if asymptomatic. Therapy other than v-nitroimidazole drugs is also an selection, only cure rates are much lower.[17]
Giardiasis [edit]
Oral metronidazole is a treatment option for giardiasis, however, the increasing incidence of nitroimidazole resistance is leading to the increased employ of other compound classes.[18]
Dracunculus [edit]
In the instance of Dracunculus medinensis (Guinea worm), metronidazole but eases worm extraction rather than killing the worm.[4]
C. difficile colitis [edit]
Initial antibiotic therapy for less-astringent Clostridioides difficile infection colitis (pseudomembranous colitis) consists of metronidazole, vancomycin, or fidaxomicin by mouth.[5] In 2017, the IDSA generally recommended vancomycin and fidaxomicin over metronidazole.[5] Vancomycin by rima oris has been shown to exist more effective in treating people with severe C. difficile colitis.[19]
E. histolytica [edit]
Entamoeba histolytica invasive amebiasis is treated with metronidazole for eradication, in combination with diloxanide to prevent recurrence.[20] Although it is generally a standard handling it is associated with some side furnishings.[21]
Preterm births [edit]
Metronidazole has too been used in women to prevent preterm nascence associated with bacterial vaginosis, amongst other risk factors including the presence of cervicovaginal fetal fibronectin (fFN). Metronidazole was ineffective in preventing preterm commitment in high-risk pregnant women (selected by history and a positive fFN exam) and, conversely, the incidence of preterm delivery was found to exist higher in women treated with metronidazole.[22]
Hypoxic radiosensitizer [edit]
In addition to its anti-biotic properties, attempts were also fabricated to use a possible radiation-sensitizing effect of metronidazole in the context of radiation therapy against hypoxic tumors.[23] Yet, the neurotoxic side effects occurring at the required dosages take prevented the widespread use of metronidazole as an adjuvant agent in radiation therapy.[24] However, other nitroimidazoles derived from metronidazole such equally nimorazole with reduced electron affinity showed less serious neuronal side effects and take found their way into radio-onological practice for head and neck tumors in some countries.[25]
Perioral dermatitis [edit]
Canadian Family unit Physician has recommended topical metronidazole equally a third-line treatment for the perioral dermatitis either forth with or without oral tetracycline or oral erythromycin equally first and second line treatment respectively.[26] [27]
Adverse effects [edit]
Common adverse drug reactions (≥1% of those treated with the drug) associated with systemic metronidazole therapy include: nausea, diarrhea, weight loss, intestinal pain, airsickness, headache, dizziness, and metal taste in the mouth. Intravenous administration is commonly associated with thrombophlebitis. Exceptional adverse effects include: hypersensitivity reactions (rash, itch, flushing, fever), headache, dizziness, vomiting, glossitis, stomatitis, night urine, and paraesthesia.[13] High doses and long-term systemic treatment with metronidazole are associated with the development of leucopenia, neutropenia, increased take chances of peripheral neuropathy, and primal nervous system toxicity.[thirteen] Mutual adverse drug reaction associated with topical metronidazole therapy include local redness, dryness and skin irritation; and eye watering (if applied about eyes).[13] [28] Metronidazole has been associated with cancer in animal studies.[29] [ failed verification ] In rare cases, it tin also cause temporary hearing loss that reverses after cessation of the handling.[30] [31]
Some prove from studies in rats indicates the possibility information technology may contribute to serotonin syndrome, although no case reports documenting this have been published to date.[32] [33]
Mutagenesis and carcinogenesis [edit]
Metronidazole is listed by the U.South. National Toxicology Program (NTP) as reasonably anticipated to be a human carcinogen.[34] Although some of the testing methods have been questioned, oral exposure has been shown to cause cancer in experimental animals and has too demonstrated some mutagenic effects in bacterial cultures.[34] [35] The relationship betwixt exposure to metronidazole and human cancer is unclear.[34] [36] I study [37] found an excess in lung cancer amidst women (even subsequently adjusting for smoking), while other studies [38] [39] [40] constitute either no increased risk, or a statistically insignificant risk.[34] [41] Metronidazole is listed as a possible carcinogen according to the World Wellness Organization (WHO) International Bureau for Research on Cancer (IARC).[42] A study in those with Crohn's disease also constitute chromosomal abnormalities in circulating lymphocytes in people treated with metronidazole.[35]
Stevens–Johnson syndrome [edit]
Metronidazole alone rarely causes Stevens–Johnson syndrome, but is reported to occur at loftier rates when combined with mebendazole.[43]
Drug interactions [edit]
Alcohol [edit]
Consuming booze while taking metronidazole has been suspected in instance reports to cause a disulfiram-like reaction with effects that can include nausea, vomiting, flushing of the peel, tachycardia, and shortness of breath.[44] People are often advised not to drink alcohol during systemic metronidazole therapy and for at least 48 hours after completion of treatment.[13] However, some studies call into question the mechanism of the interaction of booze and metronidazole,[45] [46] [47] and a possible cardinal toxic serotonin reaction for the alcohol intolerance is suggested.[32] Metronidazole is too generally thought to inhibit the liver metabolism of propylene glycol (found in some foods, medicines, and in many electronic cigarette e-liquids), thus propylene glycol may potentially have similar interaction effects with metronidazole.[ medical citation needed ]
Other drug interactions [edit]
Metronidazole is a moderate CYP2C9 inhibitor. CYP2C9 is an enzyme of cytochrome P450 family unit. Therefore, metronidazole may collaborate with medications metabolized by this enzyme.[48] [49] [50] Examples of such medications are lomitapide, warfarin, etc.[1]
Pharmacology [edit]
Machinery of activeness [edit]
Metronidazole is of the nitroimidazole course. It inhibits nucleic acid synthesis by forming nitroso radicals, which disrupt the DNA of microbial cells.[1] [51] This office only occurs when metronidazole is partially reduced, and because this reduction commonly happens just in anaerobic bacteria and protozoans, information technology has relatively little consequence upon human cells or aerobic bacteria.[52]
Pharmacokinetics [edit]
Oral metronidazole is approximately 80% bioavailable via the gut and pinnacle blood plasma concentrations occur subsequently one to two hours. Food may ho-hum down absorption merely does not diminish it. Of the circulating substance, nearly 20% is bound to plasma proteins. It penetrates well into tissues, the cerebrospinal fluid, the amniotic fluid and breast milk, too as into abscess cavities.[51]
About lx% of the metronidazole is metabolized by oxidation to the main metabolite hydroxymetronidazole and a carboxylic acrid derivative, and past glucuronidation. The metabolites show antibiotic and antiprotozoal activity in vitro.[51] Metronidazole and its metabolites are mainly excreted via the kidneys (77%) and to a lesser extent via the faeces (14%).[ane] [2] The biological half-life of metronidazole in healthy adults is eight hours, in infants during the first two months of their lives most 23 hours, and in premature babies up to 100 hours.[51]
The biological activity of hydroxymetronidazole is xxx% to 65%, and the elimination half-life is longer than that of the parent chemical compound.[53] The serum one-half-life of hydroxymetronidazole subsequently suppository was 10 hours, 19 hours after intravenous infusion, and 11 hours later on a tablet.[54]
History [edit]
The drug was initially developed by Rhône-Poulenc in the 1950s[55] and licensed to Yard.D. Searle.[56] Searle was acquired by Pfizer in 2003.[57] The original patent expired in 1982, but evergreening reformulation occurred thereafter.[58]
Brand proper name [edit]
In India, information technology is sold nether the make proper noun Metrogyl and Flagyl.[59] In Bangladesh, it is available every bit Amodis, Amotrex, Dirozyl, Filmet, Flagyl, Flamyd, Metra, Metrodol, Metryl, etc.[60]
Synthesis [edit]
two-Methylimidazole (1) may be prepared via the Debus-Radziszewski imidazole synthesis, or from ethylenediamine and acetic acid, followed by handling with lime, then Raney nickel. 2-Methylimidazole is nitrated to give 2-methyl-iv(5)-nitroimidazole (2), which is in turn alkylated with ethylene oxide or 2-chloroethanol to requite metronidazole (3):[61] [62] [63]
Veterinary apply [edit]
Metronidazole is widely used to treat infections of Giardia in dogs, cats, and other companion animals, although it does not reliably clear infection with this organism and is beingness supplanted past fenbendazole for this purpose in dogs and cats.[64] It is also used for the management of chronic inflammatory bowel disease in cats and dogs.[65] Another common usage is the treatment of systemic and/or gastrointestinal clostridial infections in horses. Metronidazole is used in the aquarium hobby to treat ornamental fish and every bit a broad-spectrum treatment for bacterial and protozoan infections in reptiles and amphibians. In general, the veterinarian community may use metronidazole for any potentially susceptible anaerobic infection. The U.South. Nutrient and Drug Assistants (FDA) suggests it only be used when necessary because it has been shown to exist carcinogenic in mice and rats, every bit well equally to prevent antimicrobial resistance.[66] [67]
References [edit]
- ^ a b c d eastward f k h "Flagyl, Flagyl ER (metronidazole) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 7 April 2014. Retrieved iii April 2014.
- ^ a b c d e f Brayfield, A, ed. (14 January 2014). "Metronidazole". Martindale: The Complete Drug Reference. Pharmaceutical Press. Retrieved three Apr 2014. [ dead link ]
- ^ Brayfield A, ed. (2017). Martindale: The Complete Drug Reference (39th ed.). London: Pharmaceutical Press. ISBN978-0-85711-309-two.
- ^ a b c d east f thou h "Metronidazole". The American Guild of Health-System Pharmacists. Archived from the original on half-dozen September 2015. Retrieved 31 July 2015.
- ^ a b c McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Bury SE, et al. (March 2018). "Clinical Exercise Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Club of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA)". Clinical Infectious Diseases. 66 (7): e1–e48. doi:10.1093/cid/cix1085. PMC6018983. PMID 29462280.
- ^ a b "Metronidazole Utilize During Pregnancy". www.drugs.com. Archived from the original on 1 January 2017. Retrieved one January 2017.
- ^ "Prophylactic in Lactation: Metronidazole and tinidazole". SPS - Specialist Pharmacy Service . Retrieved 22 Feb 2020.
- ^ Corey EJ (2013). Drug discovery practices, processes, and perspectives. Hoboken, N.J.: John Wiley & Sons. p. 27. ISBN9781118354469. Archived from the original on 8 September 2017.
- ^ World Health Organization (2019). Earth Health Organization model list of essential medicines: 21st listing 2019. Geneva: World Wellness Organisation. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA iii.0 IGO.
- ^ Schmid G (28 July 2003). "Trichomoniasis treatment in women". Archived from the original on 1 August 2015. Retrieved 1 Baronial 2015.
- ^ "The Summit 300 of 2019". ClinCalc . Retrieved 16 October 2021.
- ^ "Metronidazole - Drug Usage Statistics". ClinCalc . Retrieved 16 Oct 2021.
- ^ a b c d e f Rossi S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN978-0-9805790-9-3.
- ^ Orla Geoghegan, Christopher Eades, Luke SP Moore, Mark Gilchrist (9 Feb 2017). "Clostridium difficile: diagnosis and handling update". The Pharmaceutical Journal. Royal Pharmaceutical Society.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Dickman A (2012). Drugs in Palliative Care. OUP Oxford. p. 355. ISBN9780191636103.
- ^ Joesoef MR, Schmid GP, Hillier SL (January 1999). "Bacterial vaginosis: review of treatment options and potential clinical indications for therapy". Clinical Infectious Diseases. 28 Suppl 1: S57-65. doi:10.1086/514725. PMID 10028110.
- ^ duBouchet L, Spence MR, Rein MF, Danzig MR, McCormack WM (March 1997). "Multicenter comparing of clotrimazole vaginal tablets, oral metronidazole, and vaginal suppositories containing sulfanilamide, aminacrine hydrochloride, and allantoin in the treatment of symptomatic trichomoniasis". Sexually Transmitted Diseases. 24 (iii): 156–60. doi:ten.1097/00007435-199703000-00006. PMID 9132982. S2CID 6617019.
- ^ Leitsch D (September 2015). "Giardia lamblia". Current Tropical Medicine Reports. 2 (3): 128–135. doi:10.1007/s40475-015-0051-1. PMC4523694. PMID 26258002.
- ^ Zar FA, Bakkanagari SR, Moorthi KM, Davis MB (Baronial 2007). "A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity". Clinical Infectious Diseases. 45 (3): 302–7. doi:10.1086/519265. PMID 17599306.
- ^ Ryan KJ, Ahmad Due north, Andrew Alspaugh J, Lawrence Drew W, Lagunoff M, Pottinger P, Barth Reller L, Reller ME, Sterling CR, Weissman South (12 January 2018). Sherris medical microbiology. Ryan, Kenneth J. (Kenneth James), 1940- (Seventh ed.). New York. ISBN9781259859816. OCLC 1004770160.
- ^ Rawat A, Singh P, Jyoti A, Kaushik S, Srivastava VK (August 2020). "Averting manual: A pivotal target to manage amoebiasis". Chemical Biology & Drug Design. 96 (ii): 731–744. doi:10.1111/cbdd.13699. PMID 32356312. S2CID 218475533.
- ^ Shennan A, Crawshaw Southward, Briley A, Hawken J, Seed P, Jones M, et al. (January 2006). "General obstetrics: A randomised controlled trial of metronidazole for the prevention of preterm birth in women positive for cervicovaginal fetal fibronectin: the PREMET Study". BJOG. 113 (1): 65–74. doi:10.1111/j.1471-0528.2005.00788.x. PMID 16398774. S2CID 11366650.
- ^ D. Nori, J. Yard. Cain, B. Due south. Hilaris, Due west. B. Jones, J. L. Lewis: Metronidazole equally a radiosensitizer and high-dose radiations in advanced vulvovaginal malignancies, a airplane pilot report. In: Gynecologic Oncology. vol 16, issue 1, Baronial 1983, S. 117–128, ISSN 0090-8258, PMID 6884824.
- ^ J.R.Sarna, S.Furtado, A.Yard.Brownell: Neurologic complications of metronidazole. In: Can J Neurol Sci. vol 40, issue 6, Nov 2013, S. 768-776, PMID 24257215.
- ^ J.Overgaard, H.S.Hansen, Yard.Overgaard, L.Bastholt, A.Berthelsen, L.Specht, et al:A randomized double-blind stage III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) In: Radiother Oncol vol 46, consequence 2, May 1998, PMID 9510041.
- ^ Cheung, Melody J.; Taher, Muba; Lauzon, Gilles J. (April 2005). "Acneiform facial eruptions: a problem for immature women". Canadian Family Physician. 51: 527–533. ISSN 0008-350X. PMC1472951. PMID 15856972.
- ^ Cheung, Melody J.; Taher, Muba; Lauzon, Gilles J. (1 April 2005). "Acneiform facial eruptions: a problem for immature women". Canadian Family unit Physician. 51 (iv): 527–533. ISSN 0008-350X. PMC1472951. PMID 15856972.
- ^ Side Effects
- ^ "Flagyl metronidazole tablets label" (PDF). Archived (PDF) from the original on 22 January 2016. Retrieved five August 2015.
- ^ Iqbal SM, Murthy JG, Banerjee PK, Vishwanathan KA (April 1999). "Metronidazole ototoxicity--study of ii cases". The Journal of Laryngology and Otology. 113 (iv): 355–7. doi:10.1017/s0022215100143968. PMID 10474673.
- ^ Lawford R, Sorrell TC (Baronial 1994). "Amebic abscess of the spleen complicated by metronidazole-induced neurotoxicity: instance written report". Clinical Infectious Diseases. 19 (2): 346–8. doi:x.1093/clinids/19.2.346. PMID 7986915.
- ^ a b Karamanakos PN, Pappas P, Boumba VA, Thomas C, Malamas One thousand, Vougiouklakis T, et al. (2007). "Pharmaceutical agents known to produce disulfiram-similar reaction: effects on hepatic ethanol metabolism and brain monoamines". International Journal of Toxicology. 26 (v): 423–32. doi:10.1080/10915810701583010. PMID 17963129. S2CID 41230661.
- ^ Karamanakos PN (Nov 2008). "The possibility of serotonin syndrome brought almost by the use of metronidazole". Minerva Anestesiologica. 74 (xi): 679. PMID 18971895.
- ^ a b c d National Toxicology Programme (2016). "Metronidazole" (PDF). Study on Carcinogens (Fourteenth ed.). National Toxicology Program (NTP). Archived (PDF) from the original on 9 Feb 2020. Retrieved 9 Feb 2020.
- ^ a b "Metrogyl Metronidazole Product Data" (PDF). TGA eBusiness Services. Alphapharm Pty Express. 8 May 2013. Archived from the original on nine September 2016. Retrieved 3 Apr 2014.
- ^ Bendesky A, Menéndez D, Ostrosky-Wegman P (June 2002). "Is metronidazole carcinogenic?". Mutation Inquiry. 511 (2): 133–44. doi:10.1016/S1383-5742(02)00007-eight. PMID 12052431.
- ^ Beard CM, Noller KL, O'Fallon WM, Kurland LT, Dahlin DC (February 1988). "Cancer afterward exposure to metronidazole". Mayo Clin. Proc. 63 (ii): 147–53. doi:10.1016/s0025-6196(12)64947-vii. ISSN 0025-6196. PMID 3339906.
- ^ "Metronidazole (IARC Summary & Evaluation, Supplement7, 1987)". INCHEM2. 3 March 1998. Retrieved 12 September 2019.
- ^ Thapa PB, Whitlock JA, Brockman Worrell KG, Gideon P, Mitchel EF, Roberson P, Pais R, Ray WA (Oct 1998). "Prenatal exposure to metronidazole and risk of childhood cancer: a retrospective accomplice study of children younger than 5 years". Cancer. 83 (7): 1461–viii. doi:10.1002/(sici)1097-0142(19981001)83:vii<1461::aid-cncr25>3.0.co;two-ane. PMID 9762949.
- ^ Friedman GD, Jiang SF, Udaltsova N, Quesenberry CP, Chan J, Habel LA (Nov 2009). "Epidemiologic evaluation of pharmaceuticals with express evidence of carcinogenicity". Int. J. Cancer. 125 (ix): 2173–8. doi:10.1002/ijc.24545. ISSN 0020-7136. PMC2759691. PMID 19585498.
- ^ "Flagyl 375 U.S. Prescribing Information" (PDF). Pfizer. Archived from the original (PDF) on 7 August 2008.
- ^ "Agents Classified by the IARC Monographs, Volumes ane–124". International Bureau for Research on Cancer (IARC). 8 July 2019. Archived from the original on half-dozen September 2019. Retrieved 12 September 2019.
- ^ Chen KT, Twu SJ, Chang HJ, Lin RS (March 2003). "Outbreak of Stevens-Johnson syndrome/toxic epidermal necrolysis associated with mebendazole and metronidazole use among Filipino laborers in Taiwan". American Periodical of Public Health. 93 (3): 489–92. doi:10.2105/ajph.93.three.489. PMC1447769. PMID 12604501.
- ^ Cina SJ, Russell RA, Conradi SE (December 1996). "Sudden death due to metronidazole/ethanol interaction". The American Journal of Forensic Medicine and Pathology. 17 (iv): 343–6. doi:x.1097/00000433-199612000-00013. PMID 8947362.
- ^ Gupta NK, Woodley CL, Fried R (October 1970). "Effect of metronidazole on liver booze dehydrogenase". Biochemical Pharmacology. 19 (10): 2805–viii. doi:10.1016/0006-2952(lxx)90108-5. PMID 4320226.
- ^ Williams CS, Woodcock KR (February 2000). "Do ethanol and metronidazole collaborate to produce a disulfiram-similar reaction?". The Annals of Pharmacotherapy. 34 (2): 255–vii. doi:10.1345/aph.19118. PMID 10676835. S2CID 21151432.
the authors of all the reports presumed the metronidazole-ethanol reaction to be an established pharmacologic fact. None provided evidence that could justify their conclusions
- ^ Visapää JP, Tillonen JS, Kaihovaara PS, Salaspuro MP (June 2002). "Lack of disulfiram-like reaction with metronidazole and ethanol". The Register of Pharmacotherapy. 36 (vi): 971–4. doi:10.1345/1542-6270(2002)036<0971:lodlrw>2.0.co;2. PMID 12022894.
- ^ Flockhart DA (2007). "Drug Interactions: Cytochrome P450 Drug Interaction Table". Indiana Academy School of Medicine.
- ^ Kudo T, Endo Y, Taguchi R, Yatsu 1000, Ito One thousand (2015). "Metronidazole reduces the expression of cytochrome P450 enzymes in HepaRG cells and cryopreserved homo hepatocytes". Xenobiotica. 45 (five): 413–419. doi:10.3109/00498254.2014.990948. PMID 25470432. S2CID 26910995.
- ^ Tirkkonen T, Heikkilä P, Huupponen R, Laine K (2010). "Potential CYP2C9-mediated drug-drug interactions in hospitalized type 2 diabetes mellitus patients treated with the sulphonylureas glibenclamide, glimepiride or glipizide". Journal of Internal Medicine. 268 (4): 359–366. doi:ten.1111/j.1365-2796.2010.02257.ten. PMID 20698928. S2CID 45449460.
- ^ a b c d Haberfeld H, ed. (2020). Austria-Codex (in German language). Vienna: Österreichischer Apothekerverlag. Anaerobex-Filmtabletten.
- ^ Eisenstein BI, Schaechter M (2007). "DNA and Chromosome Mechanics". In Schaechter M, Engleberg NC, DiRita VJ, Dermody T (eds.). Schaechter's Mechanisms of Microbial Illness. Hagerstown, MD: Lippincott Williams & Wilkins. p. 28. ISBN978-0-7817-5342-v.
- ^ Lamp, K. C.; Freeman, C. D.; Klutman, N. Due east.; Lacy, One thousand. K. (1999). "Pharmacokinetics and pharmacodynamics of the nitroimidazole antimicrobials". Clinical Pharmacokinetics. 36 (5): 353–373. doi:10.2165/00003088-199936050-00004. PMID 10384859. S2CID 37891515.
- ^ Bergan, T.; Leinebo, O.; Blom-Hagen, T.; Salvesen, B. (1984). "Pharmacokinetics and bioavailability of metronidazole after tablets, suppositories and intravenous administration". Scandinavian Journal of Gastroenterology. Supplement. 91: 45–threescore. PMID 6588489.
- ^ Quirke V (29 December 2014). "Targeting the American marketplace for medicines, ca. 1950s-1970s: ICI and Rhône-Poulenc compared". Bulletin of the History of Medicine. 88 (4): 654–96. doi:ten.1353/bhm.2014.0075. PMC4335572. PMID 25557515.
- ^ "Thousand.D. SEARLE & CO. v. COMM | 88 T.C. 252 (1987) | 8otc2521326 | Leagle.com". Leagle . Retrieved 18 June 2019.
- ^ "2003:Pfizer and Pharmacia Merger". Pfizer.
- ^ Dickson Southward (July 2019). "Effect of Evergreened Reformulations on Medicaid Expenditures and Patient Access from 2008 to 2016". Journal of Managed Care & Specialty Chemist's shop. 25 (7): 780–792. doi:10.18553/jmcp.2019.18366. PMID 30799664.
- ^ "Metrogyl ER". Medical Dialogues. Retrieved one March 2021.
- ^ "Metronidazole Brands". Medex. Retrieved 24 November 2021.
- ^ Ebel K, Koehler H, Gamer AO, Jäckh R. "Imidazole and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_661.
- ^ Actor P, Chow AW, Dutko FJ, McKinlay MA. "Chemotherapeutics". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a06_173.
- ^ Kraft MY, Kochergin PM, Tsyganova AM, Shlikhunova VS (1989). "Synthesis of metronidazole from ethylenediamine". Pharmaceutical Chemistry Journal. 23 (x): 861–863. doi:ten.1007/BF00764821. S2CID 38187002.
- ^ Barr SC, Bowman DD, Heller RL (July 1994). "Efficacy of fenbendazole against giardiasis in dogs". American Journal of Veterinary Research. 55 (seven): 988–xc. PMID 7978640.
- ^ Hoskins JD (1 October 2001). "Advances in managing inflammatory bowel affliction". DVM Newsmagazine. Archived from the original on 31 December 2013. Retrieved 28 December 2013.
- ^ Plumb DC (2008). Veterinary Drug Handbook (6th ed.). Wiley. ISBN978-0-8138-2056-9.
- ^ "Metronidazole". Drugs.com. Archived from the original on 24 June 2013.
External links [edit]
- "Metronidazole". Drug Information Portal. U.S. National Library of Medicine.
- "Metronidazole". Merck manuals.
Source: https://en.wikipedia.org/wiki/Metronidazole
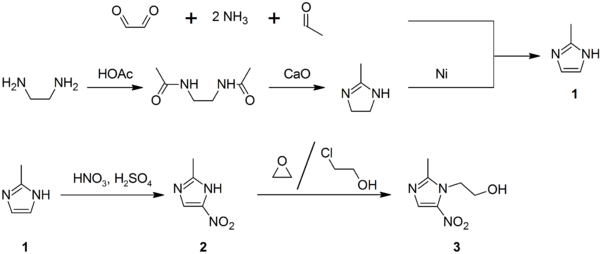
0 Response to "What if I Stop Taking Metronidazole Gel Then Started Again"
Post a Comment